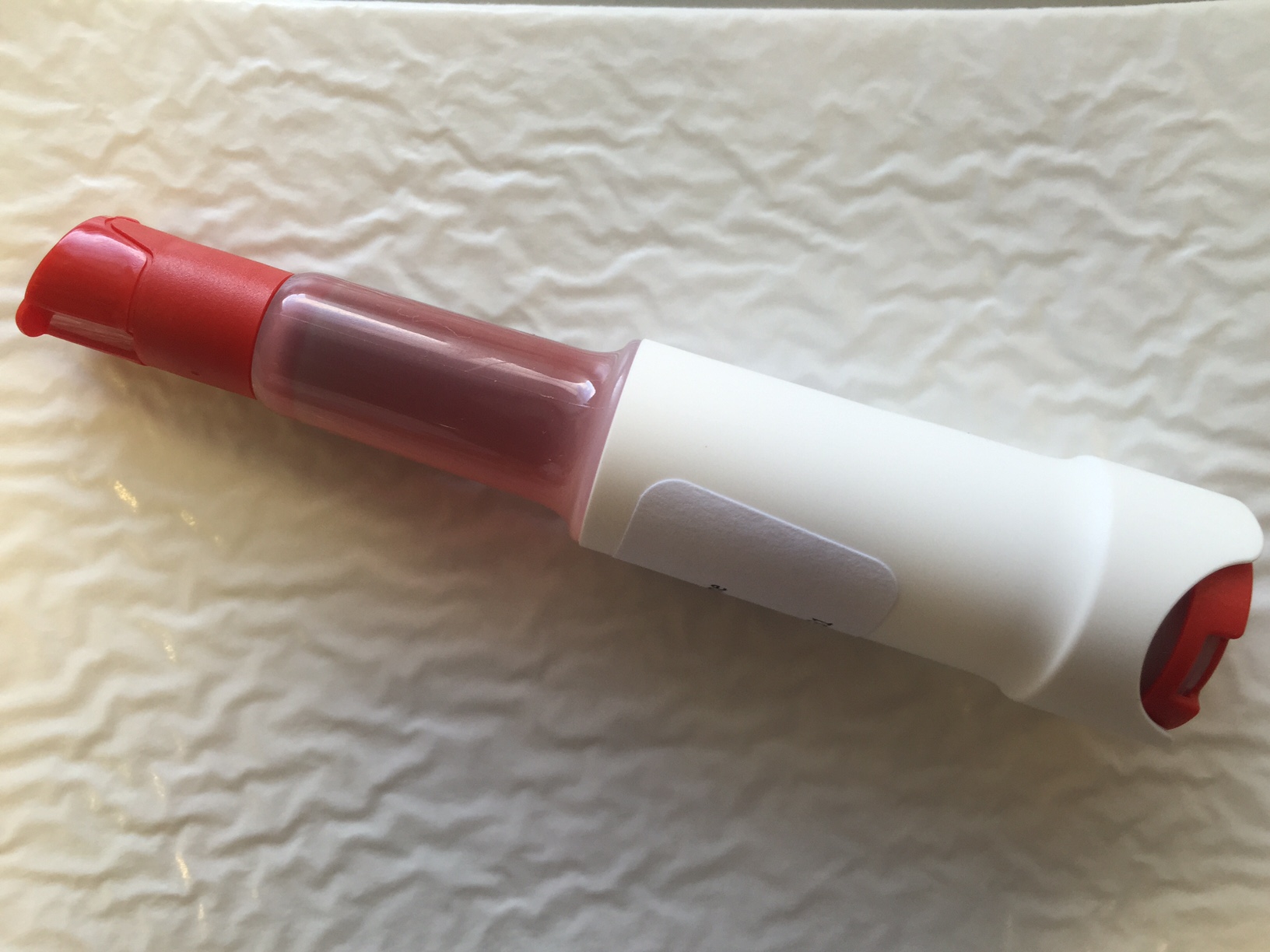
New vials for Weqas Co-oximetry scheme were recently introduced to overcome numerous issues with the previous design. We welcome your feedback on this design modification. To view a demonstration on how to use these vials, please use the link: https://www.eurotrol.com/video/acudrop

News

News
WEQAS has recently introduced a new Point of Care (POC) INR EQA control, suitable for use with the Abbott i-STAT, Roche CoaguChek XS, XS Plus & XS Pro and Siemens Xprecia Stride instruments. Point of care (POC, or near patient) testing for measurement of the international normalized ratio (INR) is
Following recent feedback from our participants, we have decided to remove the automated telephony system. A dedicated reception service is now available for all general enquiries, they will also be able to put you in touch with the right person if you are unsure of which extension you need. Our
The faecal occult blood (FOB) test detects small amounts of blood in your faeces, which would not be normally visible. FOB is vital in the diagnosis of bleeding disorders of the gut (intestine)…. For example, gastric or duodenal ulcers, ulcerative colitis, bowel polyps, and bowel (colorectal) cancer. Bowel (colorectal) cancer
EQA provision has become highly competitive and it is often difficult for Laboratories to select the right programme, with a marked lack of harmonisation of Best Practice amongst EQA Providers, it is understandable why participants are confused and often comment that they are unable to see the wood for the
The use of individual or group of biochemical markers have advanced some of the understanding on the mechanisms leading to spontaneous preterm birth. Preterm birth is a delivery that occurs at less than 37 completed weeks of gestation and it is associated with prenatal morbidity and mortality. In a point
WEQAS are pleased to release the latest issue of our Newsletter. We would like to inform you of what is happening in the quality arena, our organisation news, accreditation and service updates. In this issue, please find: * POCT Creatinine and the merits of accreditation. * WEQAS User Group Meeting
Weqas- IQC Use Weqas IQC for accurate, independent, unbiased daily monitoring. Weqas has developed a wealth of knowledge and experience in the production and distribution of high quality clinical material, representing surrogate patient samples. We are pleased to announce the launch of our Third Party Control (IQC) to assist
We are pleased to announce the launch of our Weqas Drugs of Abuse scheme (DOA), join today to take full advantage of the benefits of joining a Weqas scheme at no extra cost. This Pilot scheme will remain free of charge until April 2016, an early bird opportunity too good
Ethylene Glycol and Methanol are now available as part of our ED Toxicology scheme; these analytes have been added In response to the ‘NHS Services, Seven Day Working’ from NHS England. The Association for Clinical Biochemistry and Laboratory Medicine published guidance on the clinical biochemistry tests that may be required